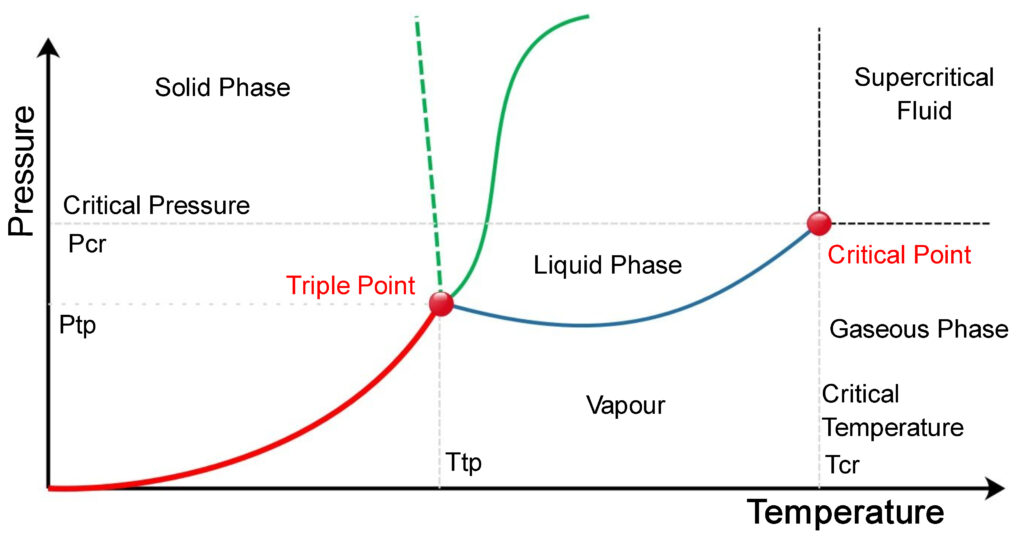
Critical Point and Triple Point
Critical Point and Triple Point are terms that refer to a certain state a substance. These terms are related to an equilibrium between phases. Phase diagrams are charts which convey information of different states of a material; Solid, Liquid, Gas, and presence of equilibrium or non-equilibrium conditions between them at different Temperatures, Pressure, and Volume.
The critical point and triple point can be located on a phase diagram. Phase diagrams having variables other than the common variables of temperature, pressure, and volume such as enthalpy, entropy, etc are also used in industries to understand or design feasible systems for turbines, compressors, air conditioners, etc. Each substance has a unique phase diagram.
The above phase diagram is called a pressure-temperature phase diagram because the chart uses pressure and temperature as its variables. The lines on the diagram represent the conditions of temperature and pressure where at least two phases of the substance co-exist in equilibrium with each other.
At least two phases mean it can be solid or liquid, liquid or gas, gas or solid, or all the three phases together. If the pressure and temperature of the material is such that the points don’t lie on the lines then the substance will exist in only one single phase, either as a solid or liquid or a gas.
The line which is clubbed between the liquid phase and the gas phase is called the vaporization curve. The line which is clubbed between the solid phase and the liquid phase is called the melting curve. The line which is clubbed between the solid phase and the gas phase is called the sublimation curve.
The critical point and triple points are labeled on the diagram. Both points act as endpoints of the vaporization curve.
Critical Point
If any substance is in a vapor-liquid equilibrium then it means the two phases; Vapour and Liquid are co-existing simultaneously. On the phase diagram, it will be indicated by any point on the vaporization curve except the endpoints.
If we increase the temperature of such a substance that is in vapor-liquid equilibrium then the pressure will increase in such a way that the state of the material will move on the vaporization curve in the direction of increasing temperature or increasing pressure. If we keep on increasing the temperature, nature will keep on increasing the pressure to maintain the state of the vapor-liquid equilibrium.
Increasing temperature does mean that the quantity of the gas may increase and the quantity of the liquid may decrease if the system is brought from one state to another but the overall quantity remains constant and the system remains in equilibrium.
While the increasing temperature we will reach a point where we won’t be able to distinguish between the liquid and gas phase. No separation between them can be seen. The temperature at this point is called critical temperature and the pressure is called critical pressure. This point on the diagram is called the critical point.
At the critical point, the heat of vaporization of the substance is zero. Vaporization indicates the conversion of a liquid to a gas and heat indicates the requirement of energy input to effect this conversion. At the critical point, no energy is to be added to convert the liquid to gas because both the phases are equally dense. Their density being equal to each other makes them indistinguishable from each other.
The combination of pressures and temperatures above the critical point gives rise to a supercritical fluid. Fisher and Widom found out that if we have a substance that is beyond its critical state then it is possible for it to be either more ‘gas-like’ or more ‘liquid-like’ depending on where it lies on the phase diagram in the supercritical region.
In essence, it means that qualitatively it is true that liquid and gas are indistinguishable but quantitatively it is possible to be either more at the liquid side or the gas side. That supercritical fluid which is more on the gas side will display properties which are slightly dominant for the gas like behavior and that supercritical fluid which is more on the liquid side will display properties that are slightly dominant for a liquid like behavior.
Triple Point
The triple point of a substance is indicated by that point on the phase diagram where the vaporization curve, melting curve, and sublimation curve intersect. If we have a substance that is at vapor-liquid equilibrium then we can reach the triple point either by decreasing the temperature or by decreasing the pressure.
At the triple point, the solid phase, liquid phase, and the gas phase all co-exist in stable equilibrium. Any small differential change in temperature and pressure will tend to make the system such that it moves away from the three-phase equilibrium system to a two-phase equilibrium system or a single-phase system.
The major use of the triple point of water was that it was used to define the kelvin. The Kelvin is the base unit of thermodynamic temperature in the International Systems of Units. Also, there are many international temperature scales that utilize the triple point of various substances to mark its points. The triple point of water was once used to define sea level on Mars.
The triple point pressure of water is the minimum pressure at which the water can exist as a liquid. For those water systems whose pressure is below the triple point pressure of water, sublimation occurs. The solid ice converts directly to water vapor without being converted to liquid.







No comments